Polyphosphate
One of the more versatile and safe to administer water stabilizer treatment available for municipal potable water systems is Polyphosphate or PO4. However, Polyphosphates can differ in the molecular structures and overall stability based on certain chemical characteristics. These characteristics are formed at the manufacturing level. Glassy Phosphates are manufactured by melting sodium phosphates of the proper composition and chilling the melts to form clear glassy products. They are non-crystalline, amorphous materials with no exact composition, and are considered to be super-cooled liquids. However, unlike other glasses, they are water soluble and, in varying degrees, exhibit certain properties of the crystalline polyphosphates.
Short Molecular Chain Polyphosphate is made near the lower end of the P2O5 range. It is used in certain applications which can benefit from its higher pH value.
Medium Molecular Chain Polyphosphates fall near the middle of the glassy composition range. It could be described as “standard polyphosphate” since it is used in in far greater quantities than any other type of polyphosphate. This molecular form of Polyphosphate allows for more available applied “chains” of PO4 to combine with natural minerals for sequestration and chelation.
Long Molecular Chain Polyphosphate is a specialty glass with a P2O5 ratio at the high end of the range and a mildly acid pH. It was invented by FMC to improve resistance to reversion or hydrolytic breakdown. This makes the Long Molecular Chain Polyphosphate especially useful in circulating water systems and liquid products which must have a long shelf or use life. It has also been shown to be a superior bonding agent in refractory manufacture.
Polyphosphates are widely used as reagents in water treatment, fertilizers, flame retardants and food additives due to their unique properties, inexpensiveness, nontoxicity and biodegradability.
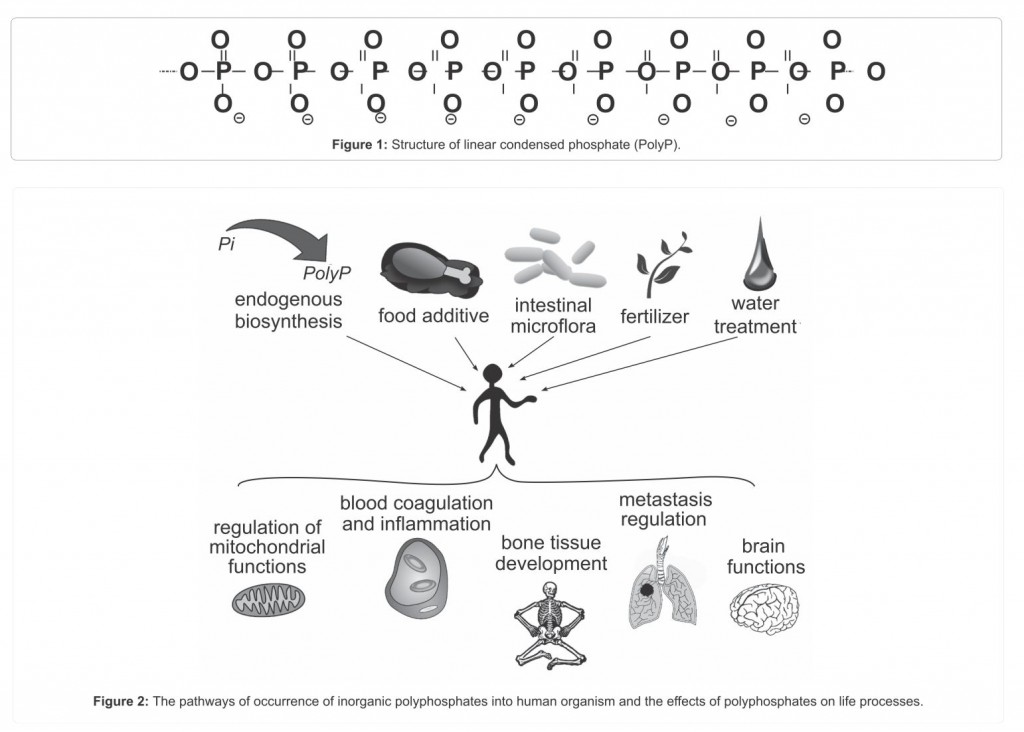
Inorganic polyphosphates are linear polymers containing a few to several hundred orthophosphate residues linked by energy-rich phosphoanhydride bonds (Figure 1)
The Figure 2 shows the main possible pathways of polyP penetration into human organism and the physiological processes they may be involved in.
HYDROLYSIS OF PHOSPHATES
The hydrolysis reaction involves breaking the P-O-P bond without permanent formation of cyclic intermediates. The hydrolytic attack is primarily at the ends of the polymeric chain and results in the formation of orthophosphate. Hydrolysis can result in loss of much of the chelation functionality of the polyphosphates. For example, while pyro-and tripolyphosphates are effective chelating agents, orthophosphates (the products of hydrolysis) possess no chelating power.
In general, the rates of hydrolysis of longer chain polyphosphates is minimized with increasing chain length due to the fact that the chain breaks down into more numerous shorter, yet viable, polyphosphate links. However, pyrophosphates and certain tri-polyphosphates show lower rates of hydrolytic breakdown than long chain polyphosphates.
