Phosphates and Polyphosphates –
Effective Corrosion Control and Sequestering Compounds for
Potable and Irrigation Water Systems.
How is Polyphosphate Manufactured?
All of the various technical and food grade phosphates and polyphosphates utilized in drinking water treatment are manufactured from phosphoric acid which is initially derived from mined processed phosphoric rock. There are two conventional processes used to create phosphoric acid – wet processing or dry furnace processing. The wet process requires sulfuric acid to digest the phosphate rock. The resultant phosphate produced by this method is supplied to the agricultural fertilizer industry. The furnace process uses heat to develop a highly purified phosphate rock inside an electric furnace to create phosphorous pentoxide. The phosphorous pentoxide is then collected and dissolved in water or a diluted solution of phosphoric acid to produce an even stronger phosphoric acid. This high purity technique is the basic component for the production of orthophosphate generally used for some potable applications. Polyphosphate, or condensed phosphate, is created under a controlled progression of temperature and time.
The Variety of Polyphosphates:
The temperature used to dehydrate the orthophosphate along with the specific “salt” added such as sodium or potassium determines the specific polyphosphate commodity. At 300° to 400° the orthophosphate is condensed to a short – two chain polyphosphate called monosodium phosphate and pyrophosphate. Depending on the specific salt used, the process creates either sodium pyrophosphate or potassium pyrophosphate.
At an even higher temperature of 400° to 500° the pyrophosphate is condensed even further to a three molecular chain length tripolyphosphate.
At temperature of 1200° and higher the tripolyphosphate condenses even further to sodium hexametaphosphate of various molecular chain lengths from 6 to 14 . The longer the process along with the increased temperature creates specific metaphosphates of medium and long chain polyphosphates.e as salts are carefully added to create various linear molecular chain lengths of 22 and higher.
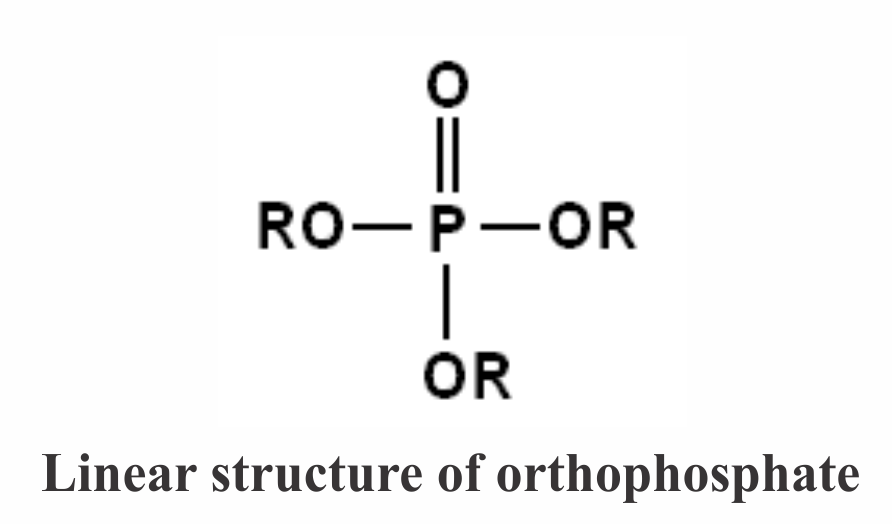
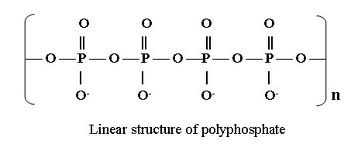
The Functions of Orthophosphates/Polyphosphates:
Polyphosphates provide many functions including the sequestering of iron and manganese and with the alkali earth metals such as calcium and magnesium. Each type of polyphosphate exhibits a different reactive sequestering rate for each of these metals/minerals. Polyphosphate also cleans or dissolves precipitated mineral scale that already exists in water distribution lines.
Hexametaphosphates (polyphosphate) inhibits scale formation caused by calcium and magnesium through sequestration and crystal growth modification at a rate 20 times more effective than the pyrophosphates. However, the pyrophosphates can sequester iron and manganese at a rate 16 times more effective than the hexametaphosphates.
Typically, in order to sequester iron, manganese and calcium it requires a 1:1 mole ratio with polyphosphate. This is generally achievable when dealing with iron and manganese that are usually in levels that are less than 5 mg/L. However, polyphosphates act as crystal modifiers that need only a fraction of that ratio to effectively modify the crystalline structure of calcium/magnesium. In theory, a hexametaphosphate dosage of 500 mg/L is required to actually sequester 200 mg/L of calcium (as calcium carbonate). However, a dosage of only 2 -4 mg/L of hexametaphosphate is all that is required to modify the crystal growth of calcium carbonate. By modifying the crystalline structure, these compounds will not precipitate into scale and actually stay in solution through repelling and suspension. This crystal growth modification function prevents the formation of mineral scale within water distribution systems. Polyphosphates will also dissolve already deposited mineral scale deposits within the system thereby increasing the carrying capacity of the water system. Polyphosphates are cathodic inhibitors that interfere with the cathodic site of the electrochemical corrosion cell formation. Anodic and cathodic electronic reactions are the two components necessary for the development of electrochemical corrosion. See: https://sperchemical.com/html/corrosion_inhibition.html This interference greatly reduces the rate of internal metallic corrosion within water distribution systems.
Orthophosphates on the other hand are used primarily as a corrosion inhibitor with its ability to create passivity film on the surface of distribution pipe. Orthophosphate is also an anodic inhibitor that interferes with the electrochemical corrosion cell formation’s anodic site that can develop on the surface of metallic pipe material. With this interference, along with the created barrier between the water and pipe surface, internal pipe corrosion is greatly reduced.
All of SPER Chemical’s water treatment formulations use the technical knowledge derived from studies that have confirmed treatment effectiveness along with only outstanding domestic sources of ingredients. We never accept less than the highest quality of ingredients – and we never accept imported ingredients from China, Mexico, or any other imported source. With the realization that our product will be added to water supplies and consumed by citizens, we do not risk the potential for contamination – especially post 9/11.
